L11 is highly conserved ribosomal protein and its interaction with the ribosomal RNA segment from 23S subunit is considered to undergo an „induced fit“-conformational adjustment of both the protein and the RNA with respect to their conformations in the unbound state (Leulliot and Varani, 2001; Draper et al., 1996). Furthermore, the conformational dynamics of L11 are thought to play an important role in the binding process.
Binding of the C-terminal domain of L11 stabilizes the tertiary structure of a compactly folded RNA domain whereas the N-terminus is implicated in the binding to the antibiotic thiostrepton (Xing and Draper, 1996). The conformation of the RNA-L11-complex is structurally well characterized (Wimberly et al., 1999, Conn et al., 1999). In addition, the conformation of the C-terminal domain of L11 from Bacillus stearothermophilus has been characterized in its RNA bound and free form by NMR (Hinck et al., 1997, Markus et al., 1997). Yet, to obtain a more detailed picture of the dynamic processes accompanying RNA-protein interactions we characterize in detail the conformation and the dynamics of the full-length protein free in solution. In particular, the relative orientation of the N- and C-terminal domain of the protein in its free form is being investigated by NMR-spectroscopy. By analysis of long-range structural information derived from heteronuclear relaxation rates and residual dipolar couplings, it can be shown that the two domains are connected by a relatively rigid poly-Proline helix, their relative flexibility is low and the prominent conformation in solution preorganizes the correct conformation in complex with RNA.
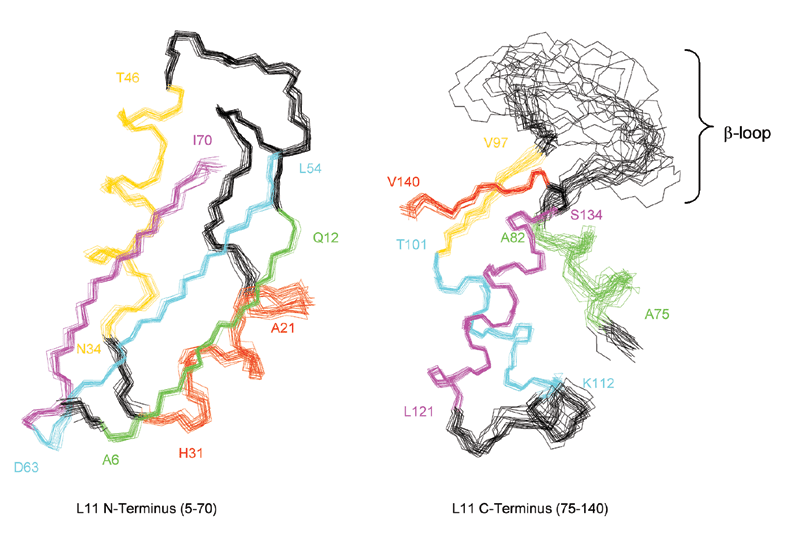
Overlay of the backbones of the 20 best structures out of 50. a) Backbone atoms of residues 5-70 that define N-terminus were fit by a least-square method. The r.m.s.d. for this superimposition is 0.28 Å for backbone atoms and 0.77 Å for all heavy atoms. b) Backbone atoms of resides 75-140 that define C-terminus. The r.m.s.d. for the superimposition of the well defined regions 97-111 and 121-140 is 0.295 Å for the backbone atoms and 0.82 Å for all heavy atoms. The beta-sheets and alpha-helices are color-coded and their first and last residues are indicated.
